Abstract
Introduction: Cyclosporine is a key drug during hematopoietic stem cell transplantation for the prevention of Graft-versus-Host disease with the potential for involvement in drug-drug interactions. We conducted this study to evaluate the effects of antifungals on cyclosporine blood levels and biavailability values.
Material and Methods: Patients who had allogeneic hematopoietic stem cell transplantation with normal kidney and liver function and no other drug-drug interactions that could affect cyclosporine blood levels were included in the study. Patients were categorized into moderate (fluconazole) and strong (voriconazole, posaconazole) CYP3A4 inhibitor antifungal users and non-azole (amphotericin B, caspofungin) users. We collected intravenous and oral cyclosporine blood concentrations and doses during cyclosporine administration route changes and evaluated the effects of them according to antifungal groups.
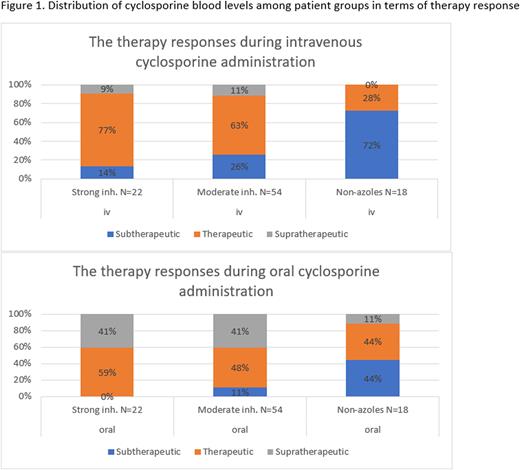
Results: We included 94 patients. The moderate and strong CYP3A4 inhibitor user numbers were 54 and 22, respectively. 18 patients with non-azole antifungal prescriptions were included. ANOVA revealed that there was a statistically significant difference in blood corrected concentrations (p<0.0001). The mean oral dose to intravenous dose ratio was 1.44 and 1.53 for the strong and moderate CYP3A4 inhibitor groups, respectively. This ratio was 1.51 for non-azole antifungal users. The median cyclosporine concentrations were higher after switching to oral cyclosporine in three antifungal groups. The highest mean cyclosporine concentration was 378,45 ng/mL and was obtained from patients with strong CYP3A4 inhibitor antifungals. The lowest mean cyclosporine concentration was observed in patients who were prescribed non-azole antifungals, which was subtherapeutic at 165,95 ng/mL. Bioavailability values were 1.17, 0.97, and 0.9 for moderate inhibitor, strong inhibitor, and non-azole users, respectively. In our study, the general oral dose to intravenous dose ratio regardless of the antifungal choice was 1.50. Despite having the lowest dose ratio (1.44), the highest bioavailability (1.17) was achieved by strong CYP3A4 inhibitor users, and nine of them experienced supratherapeutic cyclosporine levels. After applying a 1.53 dose ratio, the highest mean dose ratio among groups, the number of patients with supratherapeutic cylosporine levels increased to twenty-two from six. Eight patients with non-azole antifungal prescriptions still experienced subtherapeutic cyclosporine blood levels after the initiation of a 1.51 mean dose ratio, which suggests non-azole users may need higher oral cyclosporine doses (Figure 1).
Conclusion: Cyclosporine is a gold medicine during the allogeneic hematopoeitic stem cell trasnplantation process. Concomitant antifungal therapy affects cyclosporine blood levels during cyclosporine administration.Patients who are prescribed non-azole antifungals such as caspofungin and amphotericin B are at an increased risk of Graft-versus-Host disease due to the lack of therapeutic cyclosporine levels. Patients with azole antifungals may face cyclosporine toxicities during cyclosporine route change due to hepatic enzyme inhibitions. For patients with strong CYP3A4 inhibitor antifungals such as voriconazole and posaconazole, the oral dose to intravenous dose ratio should be adjusted carefully to avoid toxicity. Fluconazole, a moderate CYP3A4 inhibitor, causes a wide range of cyclosporine blood levels, which makes it hard to advise strict dose ratios. Because patient responses to enzyme inhibitors varied in our study, we advise taking precautions on an individual basis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal